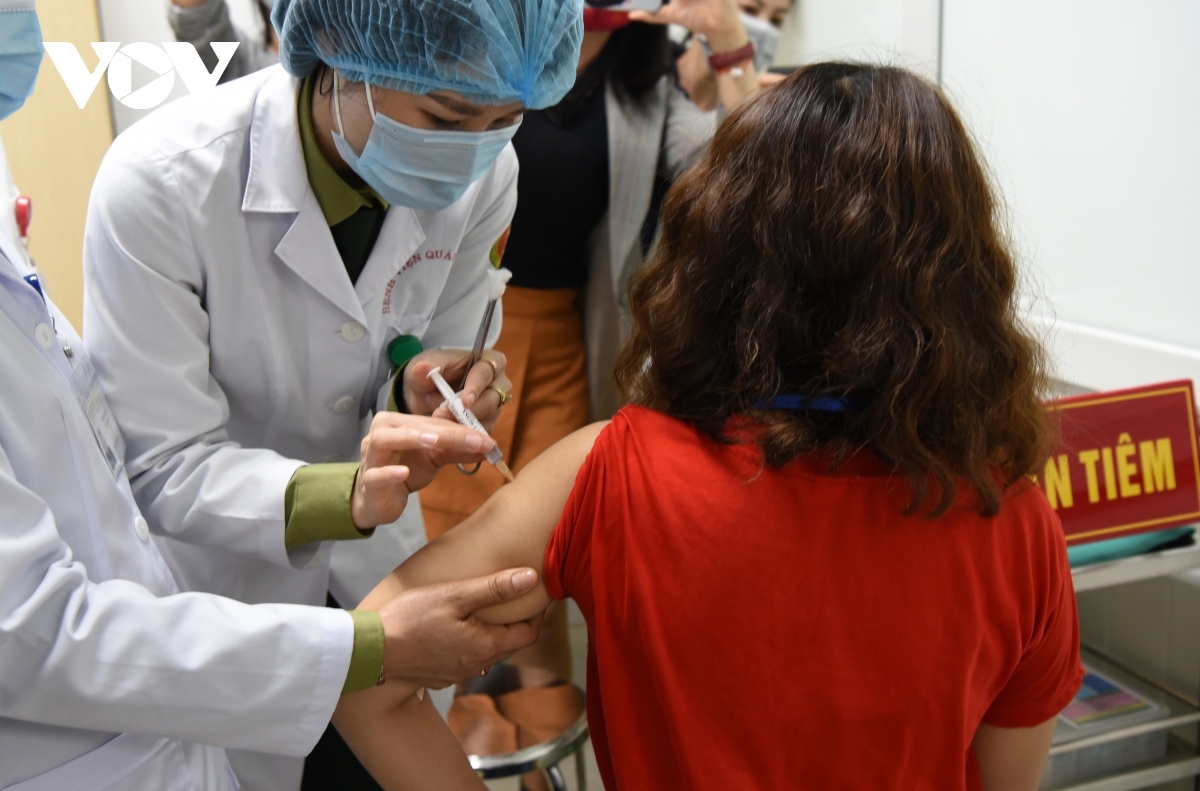
VOV.VN - The second phase of human trials for the locally-produced Nano Covax vaccine against COVID-19 were launched on the morning of February 26, following the completion of the first phase to prove the vaccine’s safety.

The testing process took place at the Vietnam Military Medical Academy in Hanoi and in the Mekong Delta province of Long An, drawing the participation of 560 volunteers aged between 18 and 65.
The volunteers are due to receive doses of 25mcg, 50mcg, and 75mcg in the second phase.
According to the Ministry of Health, the trial period will be shortened from six to three months whilst still ensuring a professional and technical process. Moving forward, the third phase is anticipated to begin in May.
Providing that the clinical trial results prove a success, the vaccine can then be produced before going on sale in the domestic market in late 2021.

Developed by Nanogen Pharmaceutical Biotechnology JSC, Nano Covax is the first domestically-made COVID-19 vaccine to start human trials, with another two produced by other manufacturers set to follow in February and March.
Vietnam is one of 40 countries to have conducted human trials on COVID-19 vaccines.
The country also has seen several other COVID-19 candidate vaccines under development. At present, the Institute of Vaccines and Medical Biologicals (IVAC), the Company for Vaccine and Biological Production No.1 (VABIOTECH), and the Centre for Research and Production of Vaccines and Biologicals (POLYVAC), are all working on their own COVID-19 vaccines.
VOV.VN - The second phase of trial injections for Nano Covax, the locally-produced COVID-19 vaccine, will be conducted on over 560 volunteers aged between 18 and 65, with the first trial shot being carried out on February 26, according to Nguyen Ngo Quang, deputy head of the Department of Science-Technology and Training.
Vietnam has completed the first phase of the human trials of Nano Covax, a homegrown COVID-19 vaccine, with the participation of 60 volunteers, the Vietnam Military Medical University said on February 8.
Bình luận
Bình luận của bạn sẽ được xét duyệt trước khi đăng