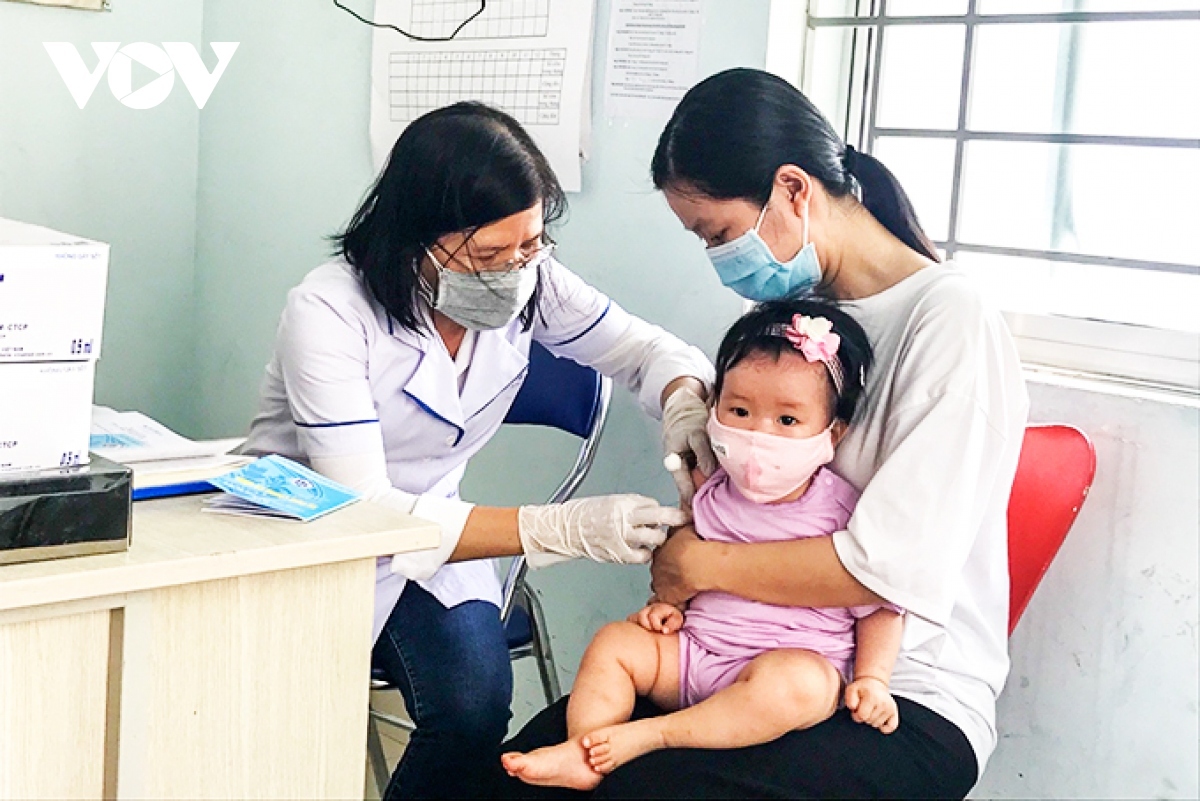
VOV.VN - As many as 40 types of vaccines and biological products have been licensed by the Ministry of Health (MoH) to be put into circulation nationwide, including three new vaccines licensed for the first time.

They include Qdenga against dengue fever, Shingrix against shingles, and Shingrix Pneumovax 23 against 23 types of pneumococcal bacteria.
All three vaccines are produced by the world’s large pharmaceutical companies, namely Takeda of Japan, GSK of Belgium, and MSD of the United States.
According to Assoc. Prof. Dr. Tran Dac Phu, former Director of the Department of Preventive Medicine, the MoH has reviewed and evaluated the safety and effectiveness of the vaccines before being put into use in the nation.
Currently, the three vaccines are in the vaccination programme that requires payment.
VOV.VN - Vietnam has for long not used the AstraZeneca vaccine against COVID-19 across the country, said a representative of the Drug Registration Division of the Drug Administration of Vietnam under the Ministry of Health.
VOV.VN - An official of the Ministry of Health has played down public worries about AstraZeneca COVID-19 vaccine side-effects after the pharmaceutical giant admitted that the vaccine may cause extremely rare side-effects such as blood clots and low platelet count.
Millions of children in Vietnam have been protected by immunisation over the past 40 years, said the World Health Organisation (WHO) and UNICEF offices in Vietnam on April 25 when they mark the World Immunisation Week from April 24-30.
Bình luận
Bình luận của bạn sẽ được xét duyệt trước khi đăng